Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. Weights of atoms and isotopes are from NIST article. The standard atomic weight is a special value of the relative atomic mass. It is defined as the 'recommended values' of relative atomic masses of sources in the local environment of the Earth's crust and atmosphere as determined by the IUPAC Commission on Atomic Weights and Isotopic Abundances (CIAAW).
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
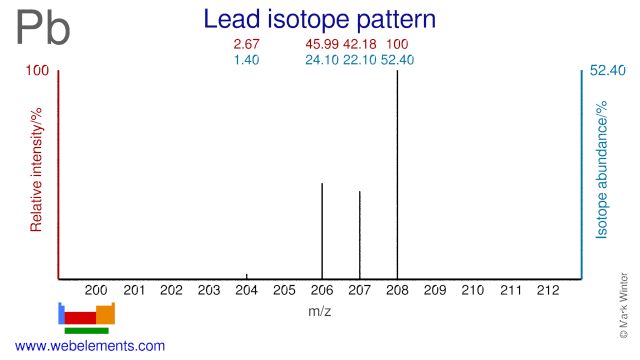
| 204Pb | 203.973 043(8) | 0.014(6) |
| 206Pb | 205.974 465(8) | 0.241(30) |
| 207Pb | 206.975 897(8) | 0.221(50) |
| 208Pb | 207.976 652(8) | 0.524(70) |
The atomic weight of lead is quite variable in nature because the three heaviest isotopes are the stableend-products of the radioactive decay of uranium (238U to 206Pb and 235U to 207Pb) and thorium (232Th to 208Pb). In fact, the variability of Ar(Pb) had been incontrovertibly shown before the discovery of isotopes andthe isotopic composition of common lead must now be regarded as a variable mixture of primeval and radiogenic components.
Recognizing this, in 1961, the Commission recommended an atomic weight of 207.19 that was based on the chemical measurements, and stated that '...it quite well represented the lead most likely to be encountered in normal laboratorywork'. However, the Commission's policy now aims for the implied range of the standard atomic weights to coverall normal sources of an element. In the 1969 report, the Commission considered natural variations in the atomic weight of lead rangingfrom 207.184 to 207.293 and recommended the value of Ar(Pb) = 207.2(1). These circumstancesjustify the annotation 'r'. In addition, the annotation 'g' warns of the existence of abnormal sources outside the implied range.
The decay of uranium and thorium to lead permits geological age determinations to be made of minerals containingthe heavy radioactive elements. Extensive use of lead over the history of mankind has led to widespread pollution, and the isotope-abundance variations reflected in the atomic weights enable historical and modern sources to be identified.
© IUPAC 2003

CIAAW
Lead
Ar(Pb) = 207.2(1) since 1969
The name derives from the Anglo-Saxon lead, which is of unknown origin. The element was knownfrom prehistoric times. The chemical symbol Pb is derived from the Latin plumbum.
Isotopic reference materials of lead.
IUPAC Commission on Isotopic Abundances and Atomic Weights.These tables are based on the 2015 table with changes from the 2015 table for the values of aluminium, argon, cobalt, gold, holmium, iridium, manganese, niobium, praseodymium, protactinium, rhodium, terbium, thulium and yttrium. See report 5 June 2018. The revised value of hafnium was reported 11 December 2019
https://www.qmul.ac.uk/sbcs/iupac/AtWt/
World Wide Web version of atomic weight data originally prepared by G. P. Moss, from a file provided by D. R. Lide.
Previous values may be consulted from the 1993 table, the 1995 table, the 1997 table, the 1999 table, the 2001 table, the 2005 table, the 2007 table, the 2009 table, the 2011 table, the 2013 table or the 2015 table.
The standard atomic weights of twelve elements having two or more stable isotopes have variability of atomic-weight values in natural terrestrial materials. These are given in table 1 below. In the other lists the values quoted are those suggested for material where the origin of the sample is unknown. For radioactive elements the isotope with the longest half-life is quoted in parenthesis. The original paper should be consulted for full details of the variation in atomic weight and the half life of the radioisotopes quoted below.
A number in parentheses indicates the uncertainty in the last digit of the atomic weight.
See below for the elements listed in Atomic Number Order or Name order.
See also a copy of the periodic table with atomic weights to five significant figures.
Table 1. List of Elements with Range of Atomic Weights.
| At No | Symbol | Name | Minimum Atomic Wt | Maximum Atomic Wt |
| 1 | H | hydrogen | 1.007 84 | 1.008 11 |
| 3 | Li | lithium | 6.938 | 6.997 |
| 5 | B | boron | 10.806 | 10.821 |
| 6 | C | carbon | 12.0096 | 12.0116 |
| 7 | N | nitrogen | 14.006 43 | 14.007 28 |
| 8 | O | oxygen | 15.999 03 | 15.999 77 |
| 12 | Mg | magnesium | 24.304 | 24.307 |
| 14 | Si | silicon | 28.084 | 28.086 |
| 16 | S | sulfur | 32.059 | 32.076 |
| 17 | Cl | chlorine | 35.446 | 35.457 |
| 18 | Ar | argon | 39.792 | 39.963 |
| 35 | Br | bromine | 79.901 | 79.907 |
| 81 | Tl | thallium | 204.382 | 204.385 |
See original paper for the range of these elements from different sources [Isotope-abundance variations and atomic weights of selected elements: 2016 (IUPAC Technical Report), Pure Appl. Chem. 2016, 88(12), 1203-1224.]
Table 2. List of Elements in Atomic Number Order.
| At No | Symbol | Name | Atomic Wt | Notes |
| 1 | H | Hydrogen | 1.008 | 3, 5 |
| 2 | He | Helium | 4.002 602(2) | 1, 2 |
| 3 | Li | Lithium | 6.94 | 3, 5 |
| 4 | Be | Beryllium | 9.012 1831(5) | |
| 5 | B | Boron | 10.81 | 3, 5 |
| 6 | C | Carbon | 12.011 | 5 |
| 7 | N | Nitrogen | 14.007 | 5 |
| 8 | O | Oxygen | 15.999 | 5 |
| 9 | F | Fluorine | 18.998 403 163(6) | |
| 10 | Ne | Neon | 20.1797(6) | 1, 3 |
| 11 | Na | Sodium | 22.989 769 28(2) | |
| 12 | Mg | Magnesium | 24.305 | 5 |
| 13 | Al | Aluminium | 26.981 5384(3) | |
| 14 | Si | Silicon | 28.085 | 5 |
| 15 | P | Phosphorus | 30.973 761 998(5) | |
| 16 | S | Sulfur | 32.06 | 5 |
| 17 | Cl | Chlorine | 35.45 | 3, 5 |
| 18 | Ar | Argon | 39.948(1) | 1, 2, 5 |
| 19 | K | Potassium | 39.0983(1) | |
| 20 | Ca | Calcium | 40.078(4) | |
| 21 | Sc | Scandium | 44.955 908(5) | |
| 22 | Ti | Titanium | 47.867(1) | |
| 23 | V | Vanadium | 50.9415(1) | |
| 24 | Cr | Chromium | 51.9961(6) | |
| 25 | Mn | Manganese | 54.938 043(2) | |
| 26 | Fe | Iron | 55.845(2) | |
| 27 | Co | Cobalt | 58.933 194(3) | |
| 28 | Ni | Nickel | 58.6934(4) | 2 |
| 29 | Cu | Copper | 63.546(3) | 2 |
| 30 | Zn | Zinc | 65.38(2) | 2 |
| 31 | Ga | Gallium | 69.723(1) | |
| 32 | Ge | Germanium | 72.630(8) | |
| 33 | As | Arsenic | 74.921 595(6) | |
| 34 | Se | Selenium | 78.971(8) | |
| 35 | Br | Bromine | 79.904 | 5 |
| 36 | Kr | Krypton | 83.798(2) | 1, 3 |
| 37 | Rb | Rubidium | 85.4678(3) | 1 |
| 38 | Sr | Strontium | 87.62(1) | 1, 2 |
| 39 | Y | Yttrium | 88.905 84(1) | |
| 40 | Zr | Zirconium | 91.224(2) | 1 |
| 41 | Nb | Niobium | 92.906 37(1) | |
| 42 | Mo | Molybdenum | 95.95(1) | 1 |
| 43 | Tc | Technetium | [97] | 4 |
| 44 | Ru | Ruthenium | 101.07(2) | 1 |
| 45 | Rh | Rhodium | 102.905 49(2) | |
| 46 | Pd | Palladium | 106.42(1) | 1 |
| 47 | Ag | Silver | 107.8682(2) | 1 |
| 48 | Cd | Cadmium | 112.414(4) | 1 |
| 49 | In | Indium | 114.818(1) | |
| 50 | Sn | Tin | 118.710(7) | 1 |
| 51 | Sb | Antimony | 121.760(1) | 1 |
| 52 | Te | Tellurium | 127.60(3) | 1 |
| 53 | I | Iodine | 126.904 47(3) | |
| 54 | Xe | Xenon | 131.293(6) | 1, 3 |
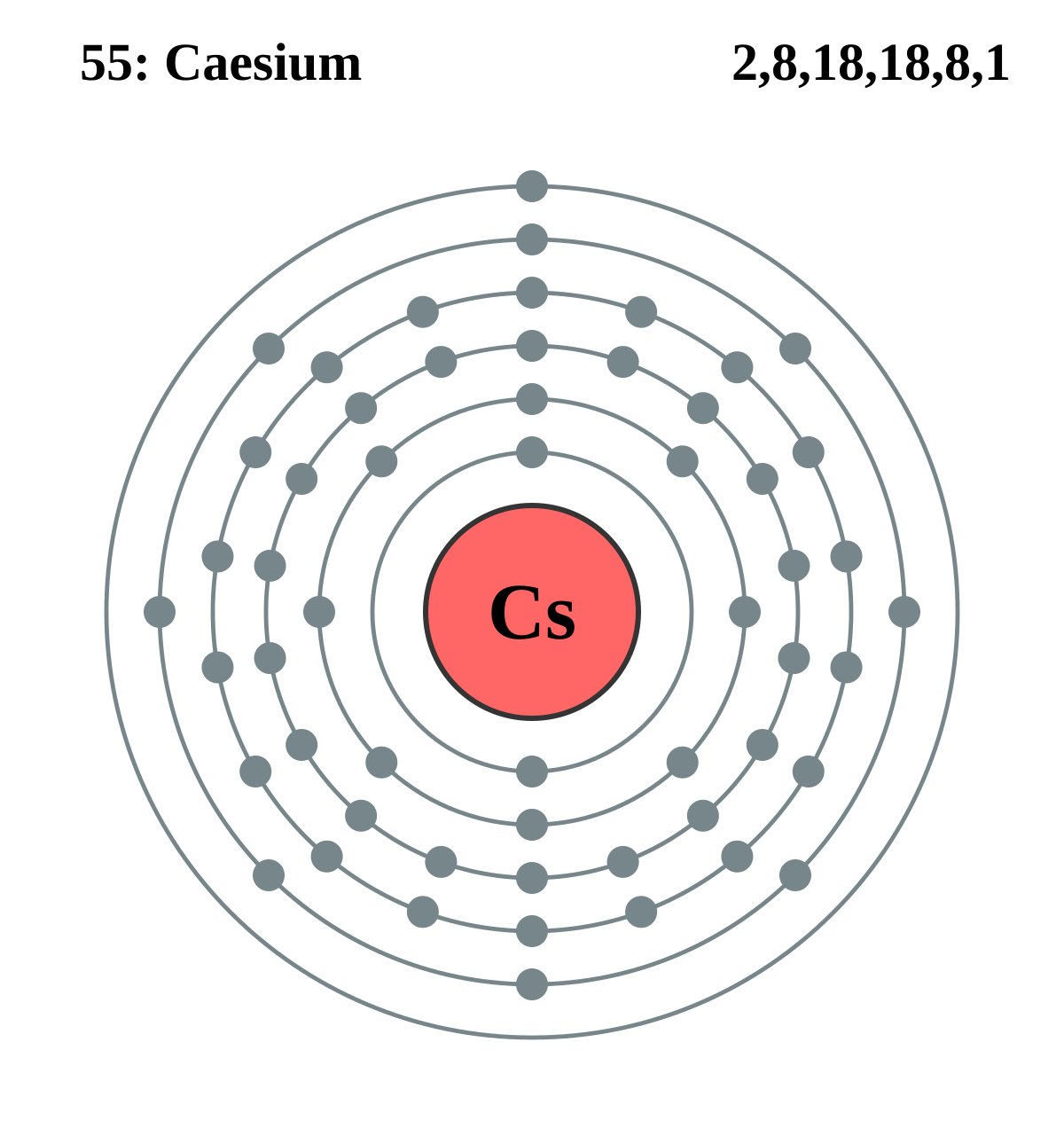
| 55 | Cs | Caesium | 132.905 451 96(6) | |
| 56 | Ba | Barium | 137.327(7) | |
| 57 | La | Lanthanum | 138.905 47(7) | 1 |
| 58 | Ce | Cerium | 140.116(1) | 1 |
| 59 | Pr | Praseodymium | 140.907 66(1) | |
| 60 | Nd | Neodymium | 144.242(3) | 1 |
| 61 | Pm | Promethium | [145] | |
| 62 | Sm | Samarium | 150.36(2) | 1 |
| 63 | Eu | Europium | 151.964(1) | 1 |
| 64 | Gd | Gadolinium | 157.25(3) | 1 |
| 65 | Tb | Terbium | 158.925 354(8) | |
| 66 | Dy | Dysprosium | 162.500(1) | 1 |
| 67 | Ho | Holmium | 164.930 328(7) | |
| 68 | Er | Erbium | 167.259(3) | 1 |
| 69 | Tm | Thulium | 168.934 218(6) | |
| 70 | Yb | Ytterbium | 173.045(10) | 1 |
| 71 | Lu | Lutetium | 174.9668(1) | 1 |
| 72 | Hf | Hafnium | 178.486(6) | |
| 73 | Ta | Tantalum | 180.947 88(2) | |
| 74 | W | Tungsten | 183.84(1) | |
| 75 | Re | Rhenium | 186.207(1) | |
| 76 | Os | Osmium | 190.23(3) | 1 |
| 77 | Ir | Iridium | 192.217(2) | |
| 78 | Pt | Platinum | 195.084(9) | |
| 79 | Au | Gold | 196.966 570(4) | |
| 80 | Hg | Mercury | 200.592(3) | |
| 81 | Tl | Thallium | 204.38 | 5 |
| 82 | Pb | Lead | 207.2(1) | 1, 2 |
| 83 | Bi | Bismuth | 208.980 40(1) | |
| 84 | Po | Polonium | [209] | 4 |
| 85 | At | Astatine | [210] | 4 |
| 86 | Rn | Radon | [222] | 4 |
| 87 | Fr | Francium | [223] | 4 |
| 88 | Ra | Radium | [226] | 4 |
| 89 | Ac | Actinium | [227] | 4 |
| 90 | Th | Thorium | 232.0377(4) | 1, 4 |
| 91 | Pa | Protactinium | 231.035 88(1) | 4 |
| 92 | U | Uranium | 238.028 91(3) | 1, 3, 4 |
| 93 | Np | Neptunium | [237] | 4 |
| 94 | Pu | Plutonium | [244] | 4 |
| 95 | Am | Americium | [243] | 4 |
| 96 | Cm | Curium | [247] | 4 |
| 97 | Bk | Berkelium | [247] | 4 |
| 98 | Cf | Californium | [251] | 4 |
| 99 | Es | Einsteinium | [252] | 4 |
| 100 | Fm | Fermium | [257] | 4 |
| 101 | Md | Mendelevium | [258] | 4 |
| 102 | No | Nobelium | [259] | 4 |
| 103 | Lr | Lawrencium | [262] | 4 |
| 104 | Rf | Rutherfordium | [267] | 4 |
| 105 | Db | Dubnium | [270] | 4 |
| 106 | Sg | Seaborgium | [269] | 4 |
| 107 | Bh | Bohrium | [270] | 4 |
| 108 | Hs | Hassium | [270] | 4 |
| 109 | Mt | Meitnerium | [278] | 4 |
| 110 | Ds | Darmstadtium | [281] | 4 |
| 111 | Rg | Roentgenium | [281] | 4 |
| 112 | Cn | Copernicium | [285] | 4 |
| 113 | Nh | Nihonium | [286] | 4 |
| 114 | Fl | Flerovium | [289] | 4 |
| 115 | Mc | Moscovium | [289] | 4 |
| 116 | Lv | Livermorium | [293] | 4 |
| 117 | Ts | Tennessine | [293] | 4 |
| 118 | Og | Oganesson | [294] | 4 |
- Geological specimens are known in which the element has an isotopic composition outside the limits for normal material. The difference between the atomic weight of the element in such specimens and that given in the Table may exceed the stated uncertainty.
- Range in isotopic composition of normal terrestrial material prevents a more precise value being given; the tabulated value should be applicable to any normal material.
- Modified isotopic compositions may be found in commercially available material because it has been subject to an undisclosed or inadvertant isotopic fractionation. Substantial deviations in atomic weight of the element from that given in the Table can occur.
- Element has no stable nuclides. The value enclosed in brackets, e.g. [209], indicates the mass number of the longest-lived isotope of the element. However three such elements (Th, Pa, and U) do have a characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.
- See table 1 for details of range and original paper for the atomic weight of the element from different sources.
Table 3. List of Elements in Name Order.
| At No | Symbol | Name | Atomic Wt | Notes |
| 89 | Ac | Actinium | [227] | 4 |
| 13 | Al | Aluminium | 26.981 5384(3) | |
| 95 | Am | Americium | [243] | 4 |
| 51 | Sb | Antimony | 121.760(1) | 1 |
| 18 | Ar | Argon | 39.948(1) | 1, 2, 5 |
| 33 | As | Arsenic | 74.921 595(6) | |
| 85 | At | Astatine | [210] | 4 |
| 56 | Ba | Barium | 137.327(7) | |
| 97 | Bk | Berkelium | [247] | 4 |
| 4 | Be | Beryllium | 9.012 1831(5) | |
| 83 | Bi | Bismuth | 208.980 40(1) | |
| 107 | Bh | Bohrium | [270] | 4 |
| 5 | B | Boron | 10.81 | 3, 5 |
| 35 | Br | Bromine | 79.904 | 5 |
| 48 | Cd | Cadmium | 112.414(4) | 1 |
| 55 | Cs | Caesium | 132.905 451 96(6) | |
| 20 | Ca | Calcium | 40.078(4) | 1 |
| 98 | Cf | Californium | [251] | 4 |
| 6 | C | Carbon | 12.011 | 5 |
| 58 | Ce | Cerium | 140.116(1) | 1 |
| 17 | Cl | Chlorine | 35.45 | 3, 5 |
| 24 | Cr | Chromium | 51.9961(6) | |
| 27 | Co | Cobalt | 58.933 194(3) | |
| 112 | Cn | Copernicium | [285] | 4 |
| 29 | Cu | Copper | 63.546(3) | 2 |
| 96 | Cm | Curium | [247] | 4 |
| 110 | Ds | Darmstadtium | [281] | 4 |
| 105 | Db | Dubnium | [270] | 4 |
| 66 | Dy | Dysprosium | 162.500(1) | 1 |
| 99 | Es | Einsteinium | [252] | 4 |
| 68 | Er | Erbium | 167.259(3) | 1 |
| 63 | Eu | Europium | 151.964(1) | 1 |
| 100 | Fm | Fermium | [257] | 4 |
| 114 | Fl | Flerovium | [289] | 4 |
| 9 | F | Fluorine | 18.998 403 163(6) | |
| 87 | Fr | Francium | [223] | 4 |
| 64 | Gd | Gadolinium | 157.25(3) | 1 |
| 31 | Ga | Gallium | 69.723(1) | |
| 32 | Ge | Germanium | 72.630(8) | |
| 79 | Au | Gold | 196.966 570(4) | |
| 72 | Hf | Hafnium | 178.486(6) | |
| 108 | Hs | Hassium | [270] | 4 |
| 2 | He | Helium | 4.002 602(2) | 1, 2 |
| 67 | Ho | Holmium | 164.930 328(7) | |
| 1 | H | Hydrogen | 1.008 | 3, 5 |
| 49 | In | Indium | 114.818(1) | |
| 53 | I | Iodine | 126.904 47(3) | |
| 77 | Ir | Iridium | 192.217(2) | |
| 26 | Fe | Iron | 55.845(2) | |
| 36 | Kr | Krypton | 83.798(2) | 1, 3 |
| 57 | La | Lanthanum | 138.905 47(7) | 1 |
| 103 | Lr | Lawrencium | [262] | 4 |
| 82 | Pb | Lead | 207.2(1) | 1, 2 |
| 3 | Li | Lithium | 6.94 | 3, 5 |
| 116 | Lv | Livermorium | [293] | 4 |
| 71 | Lu | Lutetium | 174.9668(1) | 1 |
| 12 | Mg | Magnesium | 24.305 | 5 |
| 25 | Mn | Manganese | 54.938 043(2) | |
| 109 | Mt | Meitnerium | [278] | 4 |
| 101 | Md | Mendelevium | [258] | 4 |
| 80 | Hg | Mercury | 200.592(3) | |
| 42 | Mo | Molybdenum | 95.95(1) | 1 |
| 115 | Mc | Moscovium | [289] | 4 |
| 60 | Nd | Neodymium | 144.242(3) | 1 |
| 10 | Ne | Neon | 20.1797(6) | 1, 3 |
| 93 | Np | Neptunium | [237] | 4 |
| 28 | Ni | Nickel | 58.6934(4) | |
| 113 | Nh | Nihonium | [286] | 4 |
| 41 | Nb | Niobium | 92.906 37(1) | |
| 7 | N | Nitrogen | 14.007 | 5 |
| 102 | No | Nobelium | [259] | 4 |
| 118 | Og | Oganesson | [294] | 4 |
| 76 | Os | Osmium | 190.23(3) | 1 |
| 8 | O | Oxygen | 15.999 | 5 |
| 46 | Pd | Palladium | 106.42(1) | 1 |
| 15 | P | Phosphorus | 30.973 761 998(5) | |
| 78 | Pt | Platinum | 195.084(9) | |
| 94 | Pu | Plutonium | [244] | 4 |
| 84 | Po | Polonium | [209] | 4 |
| 19 | K | Potassium | 39.0983(1) | |
| 59 | Pr | Praseodymium | 140.907 66(1) | |
| 61 | Pm | Promethium | [145] | 4 |
| 91 | Pa | Protactinium | 231.035 88(1) | 4 |
| 88 | Ra | Radium | [226] | 4 |
| 86 | Rn | Radon | [222] | 4 |
| 75 | Re | Rhenium | 186.207(1) | |
| 45 | Rh | Rhodium | 102.905 49(2) | |
| 111 | Rg | Roentgenium | [281] | 4 |
| 37 | Rb | Rubidium | 85.4678(3) | 1 |
| 44 | Ru | Ruthenium | 101.07(2) | 1 |
| 104 | Rf | Rutherfordium | [267] | 4 |
| 62 | Sm | Samarium | 150.36(2) | 1 |
| 21 | Sc | Scandium | 44.955 908(5) | |
| 106 | Sg | Seaborgium | [269] | 4 |
| 34 | Se | Selenium | 78.971(8) | |
| 14 | Si | Silicon | 28.085 | 5 |
| 47 | Ag | Silver | 107.8682(2) | 1 |
| 11 | Na | Sodium | 22.989 769 28(2) | |
| 38 | Sr | Strontium | 87.62(1) | 1, 2 |
| 16 | S | Sulfur | 32.06 | 5 |
| 73 | Ta | Tantalum | 180.947 88(2) | |
| 43 | Tc | Technetium | [97] | 4 |
| 52 | Te | Tellurium | 127.60(3) | 1 |
| 117 | Ts | Tennessine | [293] | 4 |
| 65 | Tb | Terbium | 158.925 354(8) | |
| 81 | Tl | Thallium | 204.38 | 5 |
| 90 | Th | Thorium | 232.0377(4) | 1, 4 |
| 69 | Tm | Thulium | 168.934 218(6) | |
| 50 | Sn | Tin | 118.710(7) | 1 |
| 22 | Ti | Titanium | 47.867(1) | |
| 74 | W | Tungsten | 183.84(1) | |
| 92 | U | Uranium | 238.028 91(3) | 1, 3, 4 |
| 23 | V | Vanadium | 50.9415(1) | |
| 54 | Xe | Xenon | 131.293(6) | 1, 3 |
| 70 | Yb | Ytterbium | 173.045(10) | 1 |
| 39 | Y | Yttrium | 88.905 84(1) | |
| 30 | Zn | Zinc | 65.38(2) | 2 |
| 40 | Zr | Zirconium | 91.224(2) | 1 |
- Geological specimens are known in which the element has an isotopic composition outside the limits for normal material. The difference between the atomic weight of the element in such specimens and that given in the Table may exceed the stated uncertainty.
- Range in isotopic composition of normal terrestrial material prevents a more precise value being given; the tabulated value should be applicable to any normal material.
- Modified isotopic compositions may be found in commercially available material because it has been subject to an undisclosed or inadvertant isotopic fractionation. Substantial deviations in atomic weight of the element from that given in the Table can occur.
- Element has no stable nuclides. The value enclosed in brackets, e.g. [209], indicates the mass number of the longest-lived isotope of the element. However three such elements (Th, Pa, and U) do have a characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.
- See table 1 for details of range and original paper for the atomic weight of the element from different sources.
Atomic Mass And Number Worksheet
Return to IUPAC Chemical Nomenclature home page