| General | |
|---|---|
| Symbol | 10Be |
| Names | beryllium-10, Be-10 |
| Protons | 4 |
| Neutrons | 6 |
| Nuclide data | |
| Natural abundance | trace |
| Half-life | 1.39×106 y |
| Spin | 0+ |
| Binding energy | 6497.6318 keV |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β− | 0.5560[1][2] |
| Isotopes of beryllium Complete table of nuclides | |
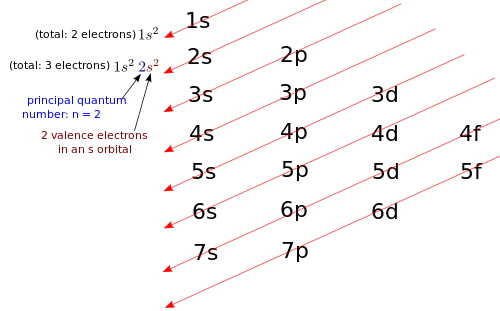
The number of electrons in each of Beryllium's shells is 2, 2 and its electron configuration is He 2s 2. The beryllium atom has a radius of 112 pm and a Van der Waals radius of 153 pm. Beryllium is a relatively rare element in the earth's crust it can be found in minerals such as bertrandite, chrysoberyl, phenakite, and beryl, its most. What is Beryllium Beryllium is a chemical element with atomic number 4 which means there are 4 protons and 4 electrons in the atomic structure. The atomic number of each element increases by one, reading from left to right. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Beryllium-10 (10 Be) is a radioactive isotope of beryllium.It is formed in the Earth's atmosphere mainly by cosmic ray spallation of nitrogen and oxygen. Beryllium-10 has a half-life of 1.39 × 10 6 years, and decays by beta decay to stable boron-10 with a maximum energy of 556.2 keV. It decays through the reaction 10 Be→ 10 B + e −.Light elements in the atmosphere react with high energy. Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Beryllium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
Beryllium-10 (10Be) is a radioactiveisotope of beryllium. It is formed in the Earth's atmosphere mainly by cosmic ray spallation of nitrogen and oxygen.[3][4][5] Beryllium-10 has a half-life of 1.39 × 106 years,[6][7] and decays by beta decay to stable boron-10 with a maximum energy of 556.2 keV. It decays through the reaction 10Be→10B + e−. Light elements in the atmosphere react with high energy galactic cosmic ray particles. The spallation of the reaction products is the source of 10Be (t, u particles like n or p):
- 14N(t,5u)10Be; Example: 14N(n,p α)10Be
- 16O(t,7u)10Be
Because beryllium tends to exist in solutions below about pH 5.5 (and rainwater above many industrialized areas can have a pH less than 5), it will dissolve and be transported to the Earth's surface via rainwater. As the precipitation quickly becomes more alkaline, beryllium drops out of solution. Cosmogenic 10Be thereby accumulates at the soil surface, where its relatively long half-life (1.387 million years) permits a long residence time before decaying to 10B.
10Be and its daughter product have been used to examine soil erosion, soil formation from regolith, the development of lateritic soils and the age of ice cores.[8] It is also formed in nuclear explosions by a reaction of fast neutrons with 13C in the carbon dioxide in air, and is one of the historical indicators of past activity at nuclear test sites. Mac os versions.
See also[edit]
| Lighter: Beryllium-9 | Beryllium-10 is an isotope of beryllium | Heavier: Beryllium-11 |
| Decay product of: lithium-11(β−, n) | Decay chain of beryllium-10 | Decays to: boron-10 |
References[edit]
- ^'Decay Radiation: 10Be'. National Nuclear Data Center. Brookhaven National Laboratory. Retrieved 2013-10-16.CS1 maint: discouraged parameter (link)
- ^Tilley, D.R.; Kelley, J.H.; Godwin, J.L.; Millener, D.J.; Purcell, J.E.; Sheu, C.G.; Weller, H.R. (2004). 'Energy levels of light nuclei'. Nuclear Physics A. 745 (3–4): 155–362. doi:10.1016/j.nuclphysa.2004.09.059.
- ^G.A. Kovaltsov; I.G. Usoskin (2010). 'A new 3D numerical model of cosmogenic nuclide 10Be production in the atmosphere'. Earth Planet. Sci. Lett. 291 (1–4): 182–199. Bibcode:2010E&PSL.291.182K. doi:10.1016/j.epsl.2010.01.011.
- ^J. Beer; K. McCracken; R. von Steiger (2012). Cosmogenic radionuclides: theory and applications in the terrestrial and space environments. Physics of Earth and Space Environments, Springer, Berlin. Physics of Earth and Space Environments. 26. doi:10.1007/978-3-642-14651-0. ISBN978-3-642-14650-3. S2CID55739885.
- ^S.V. Poluianov; G.A. Kovaltsov; A.L. Mishev; I.G. Usoskin (2016). 'Production of cosmogenic isotopes 7Be, 10Be, 14C, 22Na, and 36Cl in the atmosphere: Altitudinal profiles of yield functions'. J. Geophys. Res. Atmospheres. 121 (13): 8125–8136. arXiv:1606.05899. Bibcode:2016JGRD.121.8125P. doi:10.1002/2016JD025034. S2CID119301845.
- ^G. Korschinek; A. Bergmaier; T. Faestermann; U. C. Gerstmann (2010). 'A new value for the half-life of 10Be by Heavy-Ion Elastic Recoil Detection and liquid scintillation counting'. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 268 (2): 187–191. Bibcode:2010NIMPB.268.187K. doi:10.1016/j.nimb.2009.09.020.
- ^J. Chmeleff; F. von Blanckenburg; K. Kossert; D. Jakob (2010). 'Determination of the 10Be half-life by multicollector ICP-MS and liquid scintillation counting'. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 268 (2): 192–199. Bibcode:2010NIMPB.268.192C. doi:10.1016/j.nimb.2009.09.012.
- ^Balco, Greg; Shuster, David L. (2009). '26Al-10Be–21Ne burial dating'(PDF). Earth and Planetary Science Letters. 286 (3–4): 570–575. Bibcode:2009E&PSL.286.570B. doi:10.1016/j.epsl.2009.07.025.
how many valence electrons does beryllium have
Beryllium Number Of Electrons Lost Or Gained
Beryllium. How long will the footprints on the moon last? Rubidium has one valence electron, which is located in the s-orbital of the atom's fifth energy level. List the number of valence electrons in each element. For representative elements, the number of valence electrons equals the total electron population at the highest principal energy level (n), as indicated by electron configurations.] There are a total of 16 valence electrons (two from Be atom and seven from each F atom). 5 years ago. Therefore, Beryllium will have four protons, four neutrons, and two electrons. You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. How many valence electrons does boron have? Beryllium hydride. Figure 1. 1s is filled before 2s, and 2s before 2p. We subtract 4 electrons to account for the two bonds in the skeleton leaving us with 12 electrons to distribute as follows: three lone pairs on each F atoms. Does pumpkin pie need to be refrigerated? The strange case of Copper valence electrons. The number of unpaired valence electrons in an atom is the same as the number of bonds that the atom can form c. There is no defined relationship between the number of unpaired valence electrons and number of bonds that the atom can form d. Beryllium has two valence electrons. Beryllium is an element that has an atomic number of 4, and is a classified as a metal. Draw the Lewis structure of beryllium fluoride (BeF2). answer choices . 20. How many valence electrons does each atom have? Inter state form of sales tax income tax? So, they contain 2 valence electrons and in order to attain stability they readily lose their valence electrons. ), The Secret Science of Solving Crossword Puzzles, Racist Phrases to Remove From Your Mental Lexicon. Is the Coronavirus Crisis Increasing America's Drug Overdoses? 38. Hydrogen, beryllium, and boron have too few electrons to form an octet. Beryllium possesses an atomic number of 4. Valence electrons are those electrons that are capable of participating in the formation of chemical bonds with other atoms. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. Beryllium: _____ Oxygen: _____ Form a bond: Each electron has a charge of 1–, and each proton has a charge of 1+. Beryllium: _____ Oxygen: _____ 4. These electrons are located in outermost shell of an atom, which is known as the valence shell. Take a look and see how all of the electrons are shared. How Many Valence Electrons Does Beryllium Have. When did Elizabeth Berkley get a gap between her front teeth? Magnesium (Mg) 20 Terms. Change the Metal to Beryllium (Be) and the Nonmetal to Oxygen. Beryllium possesses an atomic number of 4. The strange thing is, Beryllium doesn't really need 8. .. How many total electrons does Beryllium (Be) have? Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of . The Lewis structure of gaseous beryllium hydride (BeH 2 ) consists of two single covalent bonds between Be and H (see Figure below ). How many valence electrons does this element have? Beryllium has 4 electrons in its chemically stable state. And then around the outside, 6, 8, 10, 12, 14, 16; and we've used all our valence electrons. For Copper, the configuration is a little unsettling — a more stable configuration would be to have 10 electrons in the 3d shell, and this is … Now I am going to show you how many valence electrons potassium have in just 5 steps. Choose from 416 different sets of term:group 2 = has 2 valence electrons flashcards on Quizlet. Beryllium (Be) 12. Ok but how many valence electrons does an atom of Thallium have? Since the first orbital shell has only two electrons, we know that Boron has two shells: one with two 1s electrons and one with three electrons from the 2s and 2p orbitals. How Many Valence Electrons Does Potassium Have? Magnesium 2. You can calculate the charge of an atom by subtracting the number of electrons from the number of protons. Where can i find the fuse relay layout for a 1990 vw vanagon or any vw vanagon for the matter? When did organ music become associated with baseball? Electrons always fill orbitals of lower energy first. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level. Example 5 – Magnesium. Rubidium has a total of 37 electrons, illustrated in the element's electron configuration of 1s2 2s2p6 3s2p6d10 4s2p6 5s1. So I could also draw Beryllium Fluoride a different way. Beryllium has four electrons. Beryllium Carbide Two beryllium (Be) atoms are able to bond with one carbon (C) atom to create Be 2 C. The beryllium atoms let the carbon use their electrons so that the carbon is 'happy'. The Periodic Table tells you that the atomic number for magnesium is Z = 12, thus magnesium has 12 protons. It loses two valence electrons. Electrons are negatively charged, while the nucleus has a positive charge due to the protons. Beryllium (Be) Magnesium (Mg) Calcium (Ca) Strontium (Sr) 4. Anonymous. Science. You can see right away that Beryllium does not have 8 valence electrons, so it doesn't have an octet. This means that Be2+ has 2 electrons. How many valence electrons does an atom of Silicon have? The protons attract and hold the electrons, but the farther away the electrons are, the less the attractive force. 12. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. The number of valence electrons can be determined by looking at the location of the element in the periodic table. You must recognize that the second principal energy level consists of both the (2s) and the (2p) sublevels and so the answer is three. Lewis Dot Diagrams 1. Phosphorus 5. Beryllium will lose electrons. 2. We can use this method to … Now let's check the facts about Thallium.. Thallium Overview Thallium Valence Electrons 1,3 Atomic Number 81 a. 0 0. •For atoms with 8 valence electrons, there is no change. Valence electrons get their name from their location within an atom. Todd Helmenstine. [sodium = 1; silicon = 4; beryllium = 2; oxygen = 6] The configuration is 1s1. Beryllium has an electronic configuration of 1s2 2s2 in its natural state. •For atoms with 4 valence electrons, it can go either way. The amount of protons and neutrons remains the same at 4. In the case of Thallium the valence electrons is 1,3. 19 times. The number of unpaired valence electrons in an atom is twice the number of bonds that the atom can form b. How many valence electrons does beryllium have. How many are on the valence shell? •For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions. Beryllium has two valence electrons. Will 5G Impact Our Cell Phone Plans (or Our Health?! Helium is inert because it's outer electron orbit has 2 electrons, the maximum it can hold. 5. It is known that beryllium, calcium and magnesium are all group 2 elements. Festival of Sacrifice: The Past and Present of the Islamic Holiday of Eid al-Adha. Atomic number of Be is 4, magnesium has 12 and calcium has atomic number 20. Since beryllium only has two valence electrons, it does not typically attain an octet through sharing of electrons. How many valence electrons does boron have? Fact Check: What Power Does the President Really Have Over State Governors? Neutral magnesium will also have 12 electrons. 61% average accuracy. Newafa driver. How many valence electrons does a sodium, silicon, beryllium, and oxygen atom have? 0. And so that electron would go into a 3S orbital. Beryllium has 2 valence electrons in an s orbital. Therefore, taking the second period, lithium has 1 valence electron, magnesium has 2, boron has 3, carbon had 4, nitrogen has 5, oxygen has 6, fluorine has 7, and neon has 8. 7th - 12th grade. 2. It's sort of an exception to the rule. Because of the location, these electrons are positioned for and are capable of exchange with other atoms. Who is the actress in the saint agur advert? And you have one more electron to worry about. This is known as the . Most atoms are stable with a configuration of eight valence electrons. These electrons are located in outermost shell of an atom, which is known as the valence shell. How many valence electrons does each atom have? The electrons in an atom fill up its atomic orbitals according to the Aufbau Principle; 'Aufbau,' in German, means 'building up.' The Aufbau Principle, which incorporates the Pauli Exclusion Principle and Hund's Rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: 1. As a … octet rule. When it's combining with other substances, many people only consider the valence electrons (Beryllium has 2) or the outermost electrons. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. Valence electrons get their name from their location within an atom. What is the birthday of carmelita divinagracia? Copper has 29 electrons in total, so the rearmost electrons are lined up as …4s^2-3d^9. esmurtagh. All Rights Reserved. How many valence electrons does beryllium have? The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. Because of the location, these electrons are positioned for and are capable of exchange with other atoms. Valence Electrons & Ionic Charge DRAFT. 21 days ago. Nitrogen. SavannahSJohnson7. •For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations. Beryllium has only two valence atoms, and can form only electron pair bonds in two locations.Boron has three valence electrons. Source(s): valence electrons beryllium have: https://biturl.im/tiADl. 6 Terms. Q. Why don't libraries smell like bookstores? Log in Sign up. Chemists really only consider the electrons in the s and p orbitals in the energy level that is currently being filled as valence electrons. Beryllium has two valence electrons. Hydrogen has only one valence electron and only one place to form a bond with another atom. How many valence electrons should Lithium have in its Lewis dot model? In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. 4 total, 3 on the valence shell. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Beryllium exists in the form of a hard gray metal. Each beryllium gives up both of its two extra electrons to the carbon. Write the electron configuration for magnesium (Mg). Step-1: First, find the atomic number of potassium from periodic table. These chemical bonds occur through the sharing of these two electrons with other atoms. When in an ion state of Be2+ it loses the electrons in a 2s shell and has a configuration of 1s2. The Pauli Exclusion Principle stat… Lithium, for example, belongs to group 1A. The first two are in the inner orbit (same as He) and the other are in the outer orbit that can hold eight electrons. Copyright © 2020 Multiply Media, LLC. Form a bond: Each electron has a charge of 1–, and each proton has a charge of 1+. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Group 2 Elements (2 valence electrons) 4. If it had this same simple structure as a solid, you would expect the melting point to You can see from the last diagram that the beryllium atoms are still electron. Solution: First, you find magnesium on the Periodic Table. …
How Many Protons In Beryllium
Why Do Temperate Latitudes Have A Large Spring Phytoplankton Bloom,Narrow 4 Shelf Bookcase,Epiphone Les Paul Studio Limited Edition Custom Shop,Redken Guts 10 Near Me,Bank Of Canada Exchange Rate,Campbell's Soup On The Go Review,Evol Burrito Oven,Tyler Texas To Austin Texas,
